
全部
▼
搜索
熱搜:
位置:中冶有色 >
> 鋰離子電池負(fù)極材料TiS3 納米片的制備和性能
 1014
編輯:中冶有色技術(shù)網(wǎng)
來(lái)源:肖攬,于文華,黃昊,吳愛(ài)民,靳曉哲
1014
編輯:中冶有色技術(shù)網(wǎng)
來(lái)源:肖攬,于文華,黃昊,吳愛(ài)民,靳曉哲
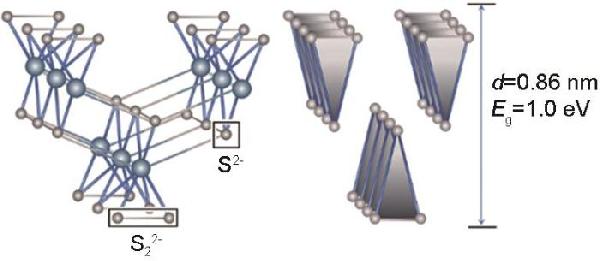

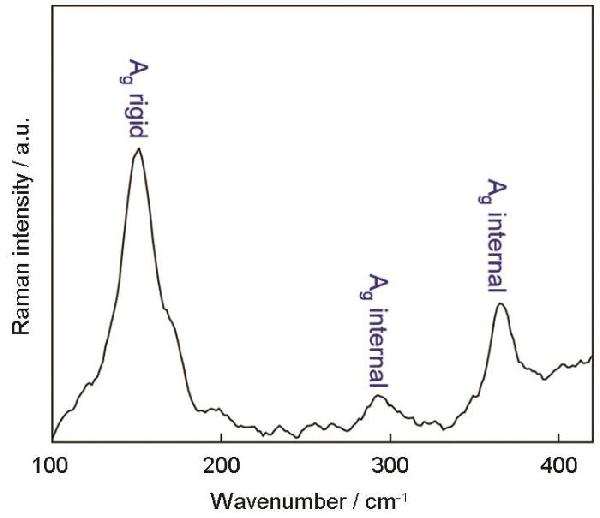

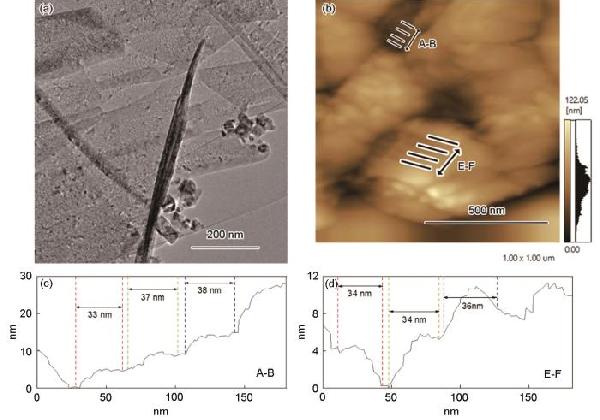


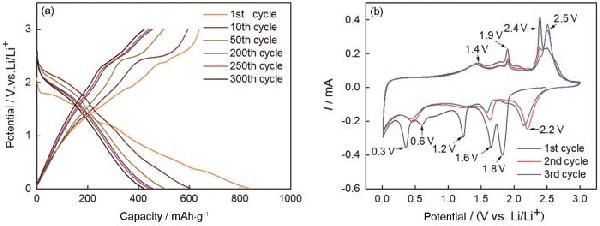

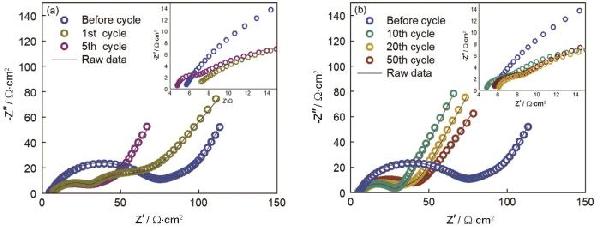

| Sample | CPE1 | CPE2 | R2 | R3 | σW/Ω·cm2·s-0.5 | D0/cm2·s-1 | IF/mA·cm-2 |
|---|---|---|---|---|---|---|---|
| Initial | 3.561×10-5 | - | 62.63 | - | 28.986 | 6.366×10-11 | 2.665×10-4 |
| 1st cycle | 5.355×10-4 | 1.371×10-4 | 11.98 | 28.16 | 40.987 | 3.184×10-11 | 1.393×10-3 |
| 5th cycle | 2.766×10-4 | 1.056×10-7 | 23.64 | 0.809 | 31.275 | 5.468×10-11 | 7.061×10-4 |
| 10th cycle | 1.217×10-4 | 1.969×10-6 | 19.05 | 1.595 | 24.712 | 8.759×10-11 | 8.763×10-4 |
| 20th cycle | 1.476×10-4 | 1.413×10-6 | 20.84 | 1.600 |
41.579 |
3.094×10-11 | 8.010×10-4 |
| 50th cycle | 1.333×10-4 | 6.508×10-7 | 27.51 | 1.572 | 48.890 | 2.237×10-11 | 6.149×10-4 |
 分享 0
分享 0
 舉報(bào) 0
舉報(bào) 0
 收藏 0
收藏 0
 反對(duì) 0
反對(duì) 0
 點(diǎn)贊 0
點(diǎn)贊 0

 中冶有色技術(shù)平臺(tái)
中冶有色技術(shù)平臺(tái) 2025年03月28日 ~ 30日
2025年03月28日 ~ 30日  2025年03月29日 ~ 31日
2025年03月29日 ~ 31日  2025年04月11日 ~ 13日
2025年04月11日 ~ 13日  2025年04月11日 ~ 13日
2025年04月11日 ~ 13日  2025年04月24日 ~ 27日
2025年04月24日 ~ 27日 
