
全部
▼
搜索
熱搜:
位置:中冶有色 >
> 離子液體輔助納米纖維素吸附劑的制備及其吸附性能
 1063
編輯:中冶有色技術(shù)網(wǎng)
來源:黃健,林春香,陳瑞英,熊萬永,溫小樂,羅鑫
1063
編輯:中冶有色技術(shù)網(wǎng)
來源:黃健,林春香,陳瑞英,熊萬永,溫小樂,羅鑫
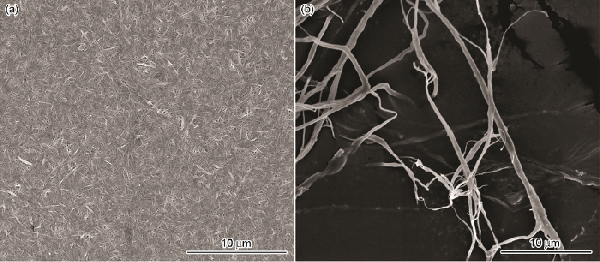
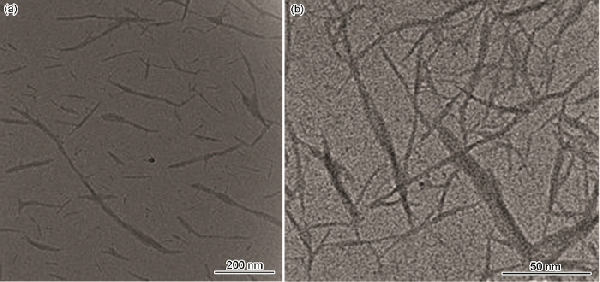




| Sample | O/% | C/% | H/% | N/% |
| NC | 51.24 | 42.65 | 6.02 | 0.37 |
| AA/AM-g-NC | 46.21 | 44.07 | 6.09 | 3.13 |
| Sample | Parameters of pore structure | ||
| Surface area/m2·g-1 |
Pore volume /cm3·g-1 |
Average pore size /nm |
|
| Ce | 3.390 | 0.0036 | 6.047 |
| NC | 13.04 | 0.013 | 4.010 |
| AA/AM-g-NC | 12.95 | 0.019 | 5.834 |
| Adsorbent | Solute | C0/mg·L-1 | qe(exp)/mg·L-1 | Ref. |
| NCC(T=35℃) | 4.80 | 2.9 | [16] | |
| 9.60 | 4.9 | |||
| 14.39 | 6.6 | |||
| NCC(T=45℃) | MB | 4.80 | 2.9 | |
| 9.60 | 5.4 | |||
| 14.39 | 6.9 | |||
| NCC(T=55℃) | 4.80 | 2.9 | ||
| 9.60 | 5.4 | |||
| 14.39 | 6.7 | |||
| 100 | 10.41 | [17] | ||
| 200 | 17.24 | |||
| NCC alginate hydrogel beads | MB | 400 | 35.28 | |
| 600 | 50.29 | |||
| 800 | 72.84 | |||
| Cellulose nanowhiskers-based polyurethane foam | MB | 50 | 43.5 | [18] |

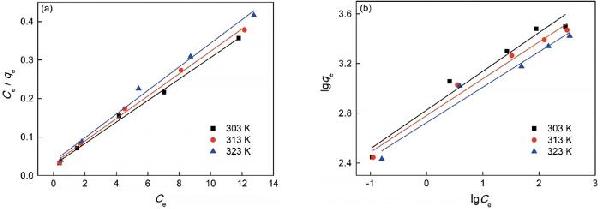
| Temperature/K | Langmuir parameters | Freundlich parameters | ||||
| Q0/mg·g-1 | b/L·mg-1 | R2 | K | 1/n | R2 | |
| 303 | 35.84 | 0.982 | 0.996 | 16.87 | 0.311 | 0.981 |
| 313 | 34.36 | 0.915 | 0.995 | 16.15 | 0.296 | 0.990 |
| 323 | 32.57 | 0.829 | 0.988 | 15.25 | 0.284 | 0.981 |
| Temperature/K | △G/kJ·mol-1 | △S/J·(mol·K)-1 | △H/kJ·mol-1 |
| 303 | -0.0459 | -0.0225 | -6.876 |
| 313 | -0.232 | -0.0212 | |
| 323 | -0.505 | -0.0197 |


| Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | ||||
| k1 | qe | R2 | k2 | qe | R2 |
| 0.030 | 56.02 | 0.935 | 0.0012 | 48.92 | 0.999 |
 分享 0
分享 0
 舉報 0
舉報 0
 收藏 0
收藏 0
 反對 0
反對 0
 點贊 0
點贊 0

 中冶有色技術(shù)平臺
中冶有色技術(shù)平臺 2025年03月25日 ~ 27日
2025年03月25日 ~ 27日  2025年03月28日 ~ 30日
2025年03月28日 ~ 30日  2025年03月28日 ~ 30日
2025年03月28日 ~ 30日  2025年04月11日 ~ 13日
2025年04月11日 ~ 13日  2025年04月27日 ~ 29日
2025年04月27日 ~ 29日 
